RBI hosts 2019 Fellows for orientation
Feb 20, 2019 — Atlanta, GA

RBI Fellows orientation
More than 50 students, faculty and staff took part in the recent RBI Fellowship orientation, hosted by the Renewable Bioproducts Institute.
The short program followed by dinner was designed as a way to introduce new RBI Fellowship recipients to the Institute, its history, strategic research areas, services and ways to get further involved with industry and other affiliated student organizations.
Meisha Shofner, RBI’s interim executive director and associate professor, MSE, said the orientation was organized to give the Fellows a better understanding of RBI, as well as provide a platform for questions with the Institute’s staff one on one.
“We are fortunate to work with a large group of talented graduate students and hope that their inclusion in RBI provides resources and opportunities that will facilitate their growth and success. This meeting was one way to let them know about ways we can support them as they navigate their graduate school careers as well as foster the community of students that has been the constant foundation of IPC, IPST and now RBI.”
RBI’s four-year fellowships are designated for Ph.D. students working on projects relating to forest biomaterials. There are four participating schools: Chemistry and Biochemistry (CHEM); Chemical and Biomolecular Engineering (ChBE); Materials Science and Engineering (MSE); and Mechanical Engineering (ME). There are currently about 40 graduate students within the program.
Learn more about RBI’s Fellowship program by visiting our website.
Kelly B. Smith, kelly.smith@gatech.edu
Can you solve the plastics problem? New prize invites ideas
Feb 13, 2019 — Atlanta, GA

Plastic is choking the ocean. A new innovation challenge looks for ideas on how to solve the pollution problem from anyone, anywhere.
Plastic is everywhere. It pools in the farthest reaches of the ocean and collects on the slopes of the highest mountains; researchers have found it in whales’ bellies and in the groundwater reserves we tap for drinking. Every day, about one megaton more is produced, enough to make almost 22 trillion water bottles—and more than 90 percent of that will never see the inside of a recycling plant.
So what should we do about it? How can we keep that plastic from drowning the planet?
Solutions to a problem as “big and knotty” as this one are going to require all hands on deck, says Valerie Craig, deputy to the chief scientist at the National Geographic Society. Good ideas could come from anyone, anywhere. So to tap the entire world’s creativity and expertise, the National Geographic Society and Sky Ocean Ventures have put together the Ocean Plastic Innovation Challenge to source ideas from around the world about how to address plastic waste.
Renewable biochemistry students tour LanzaTech biorefinery in GA
Feb 13, 2019 — Atlanta, GA

Renewable biochemistry students tour the LanzaTech biorefinery.
Students enrolled in Assistant Professor Pamela Peralta-Yahya’s (far right) Renewable Biochemistry class recently got a first-hand look at one of Georgia’s premier biorefineries, LanzaTech Freedom Pines.
The class is an elective for students pursuing a minor in Paper Science & Engineering. RBI, the administrator of the PSE program, supports these tours throughout the year in order for students to get an up-close look at facilities covering its diverse set of strategic research areas.
Brian Horton, far left, the Freedom Pines site general manager and a Georgia Tech alumni, conducted the tour. LanzaTech’s microbe has developed significant capabilities for the production of a variety of chemicals, thanks to the work of its synthetic biology team. The company has scaled this further, with the Freedom Pines site playing a core role in the development of this platform at scale.
LanzaTech produces next generation ‘advanced’ fuels by recycling waste industrial gases like those produced from steel making and other heavy industrial processes. LanzaTech takes these waste, carbon-rich gases to first make ethanol. The ethanol can be used for a range of low carbon products, including jet fuel.
At the heart of the LanzaTech process is a patented, wholly-owned microbes that convert carbon rich wastes and residues produced by industries such as steel manufacturing, oil refining and chemical production, as well as gases generated by gasification of forestry and agricultural residues, municipal waste into valuable fuel and chemical products through a process of gas fermentation.
Kelly B. Smith, kelly.smith@gatech.edu, 404.894.5301
Cultivating a Growth Mindset
Feb 13, 2019 — Atlanta, GA

Eric Taylor, recent PMML graduate
For Eric Taylor, Georgia Tech’s Professional Master’s in Manufacturing Leadership (PMML) program elevated his professional value and opened his eyes to a new take on management -- part of what he sees as a continual journey.
Taylor grew up in Southern Mississippi. While he wasn’t the first member of his family to attend college, he would prove the first to graduate -- following a period of adjustment.
“I really wasn't sure what I wanted to do after high school,” Taylor said. “I was looking at just going to work, but I did well on the ACT, so I was given a full ride to the University of Mississippi. I spent a couple of years there. I had some issues and lost that scholarship, so I went to work.”
When Taylor returned to college, it was at the University of West Alabama, and on his own dime. He worked two jobs to pay his way through school and earned a bachelor’s degree in environmental science. An internship with the chemical company, ECC International, led to a full-time job after graduation.
“I spent the next roughly 20 years in the pulp and paper industry, initially with chemical companies and eventually working for paper companies," Taylor said. "And you know, I guess I grew to a point in my life that I started to realize that a life of learning is important, and that it’s the only way to grow and develop.”
With this mindset, Taylor learned as much as he could about the paper industry. While working for the corrugated packaging company, WestRock, he also began to explore leadership and personal development opportunities. When a WestRock regional vice president suggested Georgia Tech’s PMML program, it opened an entirely new chapter in his life and career.
Mike Demaline, mike.demaline@pe.gatech.edu
Initial deadline approaching for Manufacturing Leadership program
Jan 31, 2019 — Atlanta, GA

Professional Master's of Manufacturing Leadership, Class of 2018
Four years ago, Georgia Tech launched the Professional Master’s degree in Manufacturing Leadership (PMML) as a way to energize manufacturing leadership within specific industries. The academic program aims to instruct on ways to propel your organization forward by working on real-world problems to build skills in operations and personnel management.
The two-year engineering program can be pursued one class at a time and individuals may choose from one of three areas of concentration - forest bioproducts, chemical manufacturing and discrete manufacturing.
The early deadline is approaching for the upcoming semester. Please visit the program website for information about the curriculum, admission requirements and other aspects of this advanced engineering degree or register for one of the next online Q&A sessions. Mark your calendars for the following dates of note: Early decision deadline, March 1; Final decision deadline, May 1; Program begins, August 19.
The hybrid-format blends the benefits of working face-to-face with industry experts and peers with the convenience of learning online so your employees can continue to pursue the degree while working full-time.
The Renewable Bioproducts Institute strongly supports the program – its leadership helped create it – and its potential to transform individuals, single companies and entire industries. We’ve already seen the results and heard the success stories from both graduates and their supervisors, some of whom comprise RBI’s Members Council.
Mike Demaline, mike.demaline@pe.gatech.edu
RBI spring workshop features RAPID CEO Grieco
Dec 10, 2018 — Atlanta, GA

RBI spring workshop will be held Feb. 28
The future of forest products-related industries will not solely be based upon emerging technologies, but on continuous improvement in foundational technologies and new, higher margin products from existing processes.
Join us for “Advancing Foundational Technologies to Improve the Bottom Line,” Thursday, Feb. 28, 2019, from 8 a.m. to 2 p.m. at Georgia Tech’s Renewable Bioproducts Institute on the Atlanta campus.
Delivering our keynote address will be RAPID Manufacturing Institute CEO and Georgia Tech alum William Grieco.
In 2016, the U.S. Department of Energy announced the establishment of the 10th Manufacturing USA Institute, the Rapid Advancement in Process Intensification Deployment (RAPID) Manufacturing Institute. Leveraging up to $70 million in federal funding, RAPID is focused on developing breakthrough technologies to boost energy productivity and energy efficiency by 20 percent in five years. RAPID will leverage approaches to modular chemical process intensification used in a variety of industries. Its focus on renewable bioproducts can be found here.
Grieco's senior-level innovation roles have focused on process development across multiple industries. These include the biofuels startup PetroAlgae, where he and his team built the first-of-a-kind intensive biomass production process to grow and convert aquatic plants to purified proteins and energy feedstocks. For the biopharmaceutical firm Alkermes, he spearheaded development and commercialization of the VIVITROL® and RISPERDAL®
He serves on the external advisory board for the Georgia Tech Energy Policy Innovation Center. Grieco has been a member of AIChE for 25 years, and chaired the Nanoscale Science and Engineering Forum in the mid-2000s.
The workshop will further explore how RBI is changing the way companies look at their bottom line. Listen to RBI affiliated faculty present research in areas such as black liquor separation, multi-phase forming, corrosion sciences and Factory 4.0, as well as “intelligent paper” and new products for the future of packaging and barrier protection. Find the latest draft agenda on the workshop home page as well as register here.
Attendees may also take advantage of one-on-one interactions with graduate students as they share their research a poster session and luncheon at the Paper Tricentennial Building.

RAPID Manufacturing Institute CEO Bill Grieco
Kelly B. Smith, kelly.smith@gatech.edu
Solving a 75-Year-Old Mystery Might Provide a New Source of Farm Fertilizer
Nov 27, 2018 — Atlanta, GA

Titanium dioxide, also known as titania, has photocatalytic properties that allow it to react with nitrogen. (Credit: Rob Felt, Georgia Tech)
The solution to a 75-year-old materials mystery might one day allow farmers in developing nations to produce their own fertilizer on demand, using sunlight and nitrogen from the air.
Thanks to a specialized X-ray source at Lawrence Berkeley National Laboratory, researchers at the Georgia Institute of Technology have confirmed the existence of a long-hypothesized interaction between nitrogen and titanium dioxide (TiO2) – a common photoactive material also known as titania – in the presence of light. The catalytic reaction is believed to use carbon atoms found as contaminants on the titania.
If the nitrogen-fixing reaction can be scaled up, it might one day help power clean farm-scale fertilizer production that could reduce dependence on capital-intensive centralized production facilities and costly distribution systems that drive up costs for farmers in isolated areas of the world. Most of the world’s fertilizer is now made using ammonia produced by the Haber-Bosch process, which requires large amounts of natural gas.
“In the United States, we have an excellent production and distribution system for fertilizer. However, many countries are not able to afford to build Haber-Bosch plants, and may not even have adequate transportation infrastructure to import fertilizers. For these regions, photocatalytic nitrogen fixation might be useful for on-demand fertilizer production,” said Marta Hatzell, an assistant professor in Georgia Tech’s Woodruff School of Mechanical Engineering. “Ultimately, this might be a low-cost process that could make fertilizer-based nutrients available to a broader array of farmers.”
Hatzell and collaborator Andrew Medford, an assistant professor in Georgia Tech’s School of Chemical and Biomolecular Engineering, are working with scientists at the International Fertilizer Development Center (IFDC) to study the potential impacts of the reaction process. The research was reported October 29 in the Journal of the American Chemical Society.
The research began more than two years ago when Hatzell and Medford began collaborating on a materials mystery that originated with a 1941 paper published by Seshacharyulu Dhar, an Indian soil scientist who reported observing an increase in ammonia emitted from compost subjected to light. Dhar suggested that a photocatalytic reaction with minerals in the compost could be responsible for the ammonia.
Since that paper, other researchers have reported nitrogen fixation on titania and ammonia production, but the results have not been consistently confirmed experimentally.
Medford, a theoretician, worked with graduate research assistant Benjamin Comer to model the chemical pathways that would be needed to fix nitrogen on titania to potentially create ammonia using additional reactions. The calculations suggested the proposed process was highly unlikely on pure titania, and the researchers failed to win a grant they had proposed to use to study the mysterious process. However, they were awarded experimental time on the Advanced Light Source at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory, which allowed them to finally test a key component of the hypothesis.
Specialized equipment at the lab allowed Hatzell and graduate student Yu-Hsuan Liu to use X-ray photoelectron spectroscopy (XPS) to examine the surface of titania as nitrogen, water and oxygen interacted with the surfaces under near-ambient pressure in the dark and in the light. At first, the researchers saw no photochemical nitrogen fixation, but as the experiments continued, they observed a unique interaction between nitrogen and titania when light was directed at the minerals surface.
What accounted for the initial lack of results? Hatzell and Medford believe that surface contamination with carbon – likely from a hydrocarbon – is a necessary part of the catalytic process for nitrogen reduction on the titania. “Prior to testing, the samples are cleaned to remove nearly all the trace carbon from the surface, however during experiments carbon from various sources (gases and the vacuum chamber) can introduce trace amount of carbon back onto the sample,” Hatzell explained. “What we observed was that reduced nitrogen species only were detected if there was a degree of carbon on the sample.”
The hydrocarbon contamination hypothesis would explain why earlier research had provided inconsistent results. Carbon is always present at trace levels on titania, but getting the right amount and type may be key to making the hypothesized reaction work.
“We think this explains the puzzling results that had been reported in the literature, and we hope it gives insights into how to engineer new catalysts using this 75-year-old mystery,” Medford said. “Often the best catalysts are materials that are very pristine and made in a clean room. Here you have just the opposite – this reaction actually needs the impurities, which could be beneficial for sustainable applications in farming.”
The researchers hope to experimentally confirm the role of carbon with upcoming tests at Pacific Northwest National Laboratory (PNNL), which will allow them to directly probe the carbon during the photocatalytic nitrogen fixation process. They also hope to learn more about the catalytic mechanism so that they can better control the reaction to improve efficiency, which is currently less than one percent.
The research reported in the journal did not measure ammonia, but Hatzell and her students have since detected it in lab scale tests. Because the ammonia is currently produced at such low levels, the researchers had to take precautions to avoid ammonia-based contamination. “Even tape used on equipment can create small quantities of ammonia that can affect the measurements,” Medford added.
Though the amounts of ammonia produced by the reaction are currently low, Hatzell and Medford believe that with process improvements, the advantages of on-site fertilizer production under benign conditions could overcome that limitation.
“While this may sound ridiculous from a practical perspective at first, if you actually look at the needs of the problem and the fact that sunlight and nitrogen from the air are free, on a cost basis it starts to look more interesting,” Medford said. “If you could operate a small-scale ammonia production facility with enough capacity for one farm, you have immediately made a difference.”
Hatzell credits cutting-edge surface science with finally providing an explanation to the mystery.
“Since earlier investigators looked at this, there have been significant advances made in the area of measurement and surface science,” she said. “Most surface science measurements require the use of ultra-high vacuum conditions which do not mimic the catalytic environment you aim to investigate. The near ambient pressure XPS at Lawrence Berkeley National lab, allowed us to take a step closer to observing this reaction in its native environment.”
The research was supported by startup funds from Georgia Tech to Hatzell and Medford, and by Georgia Tech’s Serve-Learn-Sustain initiative. The effort also received a boost from Georgia Tech’s Technological Innovation: Generating Economic Results (TI:GER®) program, which supported research into potential stakeholders for scale-up of the process.
In addition to those already mentioned, the research included Marm B. Dixit and Kelsey B. Hatzell from Vanderbilt University and Yifan Ye and Ethan J. Crumlin from Lawrence Berkeley Laboratory.
This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract number DE-AC02-05CH11231.
CITATION: Benjamin M. Comer, et al., “The Role of Adventitious Carbon in Photo-catalytic Nitrogen Fixation by Titania,” (Journal of American Chemical Society, 2018). http://dx.doi.org/10.1021/jacs.8b08464
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (404-894-6986) (jtoon@gatech.edu).
Writer: John Toon

Georgia Tech graduate research assistant Yu-Hsuan Liu places a sample of titanium dioxide into test equipment in the laboratory of assistant professor Marta Hatzell. (Credit: Rob Felt, Georgia Tech)

Georgia Tech graduate research assistant Yu-Hsuan Liu places a sample of titanium dioxide into test equipment in the laboratory of assistant professor Marta Hatzell. (Credit: Rob Felt, Georgia Tech)
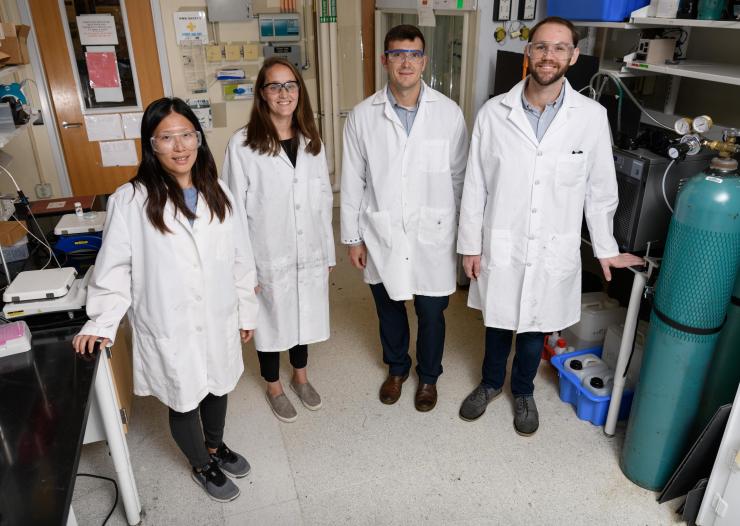
Georgia Tech researchers have recently confirmed photo-induced interaction between a titania surface and nitrogen. Shown (l-r) are graduate research assistant Yu-Hsuan Liu, assistant professor Marta Hatzell, assistant professor Andrew Medford and graduate research assistant Benjamin Comer. (Credit: Rob Felt, Georgia Tech)
John Toon
Research News
(404) 894-6986
Microfluidic Molecular Exchanger Helps Control Therapeutic Cell Manufacturing
Oct 10, 2018 — Atlanta, GA

Researchers have demonstrated an integrated technique for monitoring specific biomolecules – such as growth factors – that could indicate the health of living cell cultures produced for the burgeoning field of cell-based therapeutics.
Using microfluidic technology to advance the preparation of samples from the chemically complex bioreactor environment, the researchers have harnessed electrospray ionization mass spectrometry (ESI-MS) to provide online monitoring that they believe will provide for therapeutic cell production the kind of precision quality control that has revolutionized other manufacturing processes.
“The way that the production of cell therapeutics is done today is very much an art,” said Andrei Fedorov, Woodruff Professor in the George W. Woodruff School of Mechanical Engineering at the Georgia Institute of Technology. “Process control must evolve very quickly to support the therapeutic applications that are emerging from bench science today. We think this technology will help us reach the goal of making these exciting cell-based therapies widely available.”
By measuring very low concentrations of specific compounds secreted or excreted by cells, the technique could also help identify which biomolecules – of widely varying sizes – should be monitored to guide the control of cell health. Ultimately, the researchers hope to integrate their label-free monitoring directly into high-volume bioreactors that will produce cells in quantities large enough to make the new therapies available at a reasonable cost and consistent quality.
Development of the Dynamic Mass Spectrometry Probe (DMSP) was supported by the National Science Foundation (NSF) Engineering Research Center for Cell Manufacturing Technologies (CMaT), which is headquartered at Georgia Tech. The work was reported September 10 in the journal Biotechnology and Bioengineering.
Traditional ESI-MS techniques have revolutionized analytical chemistry by allowing precise identification of complex biological compounds. Because of complex sample preparation requirements, existing approaches to ESI-MS require too much time to be useful for continuous monitoring of cell growth in bioreactors, where maintaining narrow parameters for specific indicators of cellular health is critical. Biological samples also contain salts, which must be removed before introduction into the ESI-MS system.
To accelerate the analytical process, Fedorov and a team that included graduate research assistant Mason Chilmonczyk and research engineer Peter Kottke used microfluidic technology to help separate compounds of interest from the salts. Salt removal uses a monolithic device in which a size-selective membrane with nanoscale pores is placed between two fluid flows, one the chemically complex sample drawn from the bioreactors and the other salt-free water with conditioning compounds.
The smaller salt molecules readily diffuse out of the sampled bioreactor flow through the nanopores, while the larger biomolecules mostly remain for the subsequent ESI-MS analysis. Meanwhile, chemical additives are at the same time introduced into the sample mixture through the same membrane nanopores to enhance ionization of the target biomolecules in the sampled mixture for improved ESI-MS analysis.
“We have used advanced microfabrication techniques to create a microfluidic device that will be able to treat samples in less than a minute,” said Chilmonczyk. “Traditional sample preparation can require hours to days.”
The process can currently remove as much as 99 percent of the salt, while retaining 80 percent of the biomolecules. Introduction of the conditioning chemicals allows the molecules to accept a greater charge, improving the capability of the mass spectrometer to detect low concentration biomolecules, and to measure large molecules.
“We can detect really high molecular weight molecules that the mass spectrometer normally wouldn’t be able to detect,” Fedorov said. “The size difference in the molecules of interest can be dramatic, so the improvement in the limit of detection across a broad range of analyte molecular weights will allow this technique to be more useful in cell manufacturing.”
Because they use state of the art microfabrication techniques, the DMSP devices can be mass produced, allowing sampling to be scaled up to include multiple bioreactors at low cost. The small size of the device channels – which are just five microns tall – allows the system to produce results with samples as small as 20 nanoliters – with the potential for reducing that to as little as a single nanoliter.
“We need to monitor small concentrations of large biomolecules in this messy environment in a production line in such a way that we can check at any point how the cells are doing,” Fedorov said. “This system could continuously monitor whether certain molecules are excreted or secreted at a reduced or increased rate. By correlating these measurements with cell health and potency, we could improve the manufacturing process.”
Before the analytical techniques can be applied to quality control, the researchers must first identify biomolecules that indicate health of the growing cells. By sampling the bioreactor content locally in the immediate vicinity of cells and allowing identification of very small quantities of biochemicals, the DMSP technology can help researchers identify changes in molecular concentrations – which range from pico-molar to micro-molar – that may indicate the state of cells in the bioreactors. This would prompt adjustment of conditions in a bioreactor just in time to return to the state of healthy cell growth.
“In this situation, we often can’t see the trees for the forest,” said Fedorov. “There is a lot of material available, but we are looking for just a handful of individual trees that indicate the health of the cells. Because the forest is overgrown, the few selected trees we need to examine are hard to find. This is a grand challenge technologically.”
The research team also included Research Scientist Hazel Stevens and Professor Robert Guldberg, who is now at the University of Oregon.
CITATION: Mason A. Chilmonczyk, Peter A. Kottke, Hazel Y. Stevens, Robert E. Guldberg and Andrei G. Fedorov, “Dynamic Mass Spectrometry Probe (DMSP) for ESI?MS Monitoring of Bioreactors for Therapeutic Cell Manufacturing,” (Biotechnology and Bioengineering, 2018). https://dx.doi.org/10.1002/bit.26832
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (404-894-6986) (jtoon@gatech.edu).
Writer: John Toon
John Toon
Research News
(404) 894-6986
