Jan. 13, 2025
Georgia Tech researchers have developed biosensors with advanced sleuthing skills and the technology may revolutionize cancer detection and monitoring.
The tiny detectives can identify key biological markers using logical reasoning inspired by the “AND” function in computers — like, when you need your username and password to log in. And unlike traditional biosensors comprised of genetic materials — cells, bits of DNA — these are made of manufactured molecules.
These new biosensors are more precise and simpler to manufacture, reducing the number of false positives and making them more practical for clinical use. And because the sensors are cell-free, there’s a reduced risk for immunogenic side effects.
“We think the accuracy and simplicity of our biosensors will lead to accessible, personalized, and effective treatments, ultimately saving lives,” said Gabe Kwong, associate professor and Robert A. Milton Endowed Chair in the Wallace H. Coulter Department of Biomedical Engineering, who led the study, published this month in Nature Nanotechnology.
Breaking With Tradition
The researchers set out to address the limitations in current biosensors for cancer, like the ones designed for CAR-T cells to allow them to recognize tumor cells. These advanced biosensors are made of genetic material, and there is growing interest to reduce the potential for off-target toxicity by using Boolean “AND-gate” computer logic. That means they’re designed to release a signal only when two specific conditions are met.
“Traditionally, these biosensors involve genetic engineering using cell-based systems, which is a complex, time-consuming, and expensive process,” said Kwong.
So, his team developed biosensors made of iron oxide nanoparticles and special molecules called cyclic peptides. Synthesizing nanomaterials and peptides is a simpler, less costly process than genetic engineering, according to Kwong, “which means we can likely achieve large-scale, economical production of high-precision biosensors.”
Unlocking the AND-gate
Biosensors detect cancer signals and track treatment progress by turning biological signals into readable outputs for doctors. With AND-gate logic, two distinct inputs are required for an output.
Accordingly, the researchers engineered cyclic peptides — small amino acid chains — to respond only when they encounter two specific types of enzymes, proteases called granzyme B (secreted by the immune system) and matrix metalloproteinase (from cancer cells). The peptides generate a signal when both proteases are present and active.
Think of a high-security lock that needs two unique keys to open. In this scenario, the peptides are the lock, activating the sensor signal only when cancer is present and being confronted by the immune system.
“Our peptides allow for greater accuracy in detecting cancer activity,” said the study’s lead author, Anirudh Sivakumar, a postdoctoral researcher in Kwong’s Laboratory for Synthetic Immunity. “It’s very specific, which is important for knowing when immune cells are targeting and killing tumor cells.”
Super Specific
In animal studies, the biosensors successfully distinguished between tumors that responded to a common cancer treatment called immune checkpoint blockade therapy — ICBT, which enhances the immune system — from tumors that resisted treatment.
During these tests, the sensors also demonstrated their ability to avoid false signals from other, unrelated health issues, such as when the immune system confronted a flu infection in the lungs, away from the tumor.
“This level of specificity can be game changing,” Kwong said. “Imagine being able to identify which patients are responding to the therapy early in their treatment. That would save time and improve patient outcomes.”
The first step toward this simpler, precise form of cancer diagnostics began with an ambitious but humble ($50,000) seed grant from the Petit Institute for Bioengineering and Bioscience five years ago for a collaboration between Kwong’s lab and the lab of M.G. Finn, professor and chair in the School of Chemistry and Biochemistry.
It evolved into a multi-institutional project supported by grants from the National Science Foundation and National Institutes of Health that included researchers from the University of California-Riverside, as well as Georgia Tech faculty researchers Finn and Peng Qiu, associate professor in the Coulter Department.
“The progression of the research, from an initial seed grant all the way to animal studies, was very smooth,” Kwong said. “Ultimately, a collaborative, multidisciplinary effort turned our early vision into something that could have a great impact in healthcare.”
Citation: Anirudh Sivakumar, Hathaichanok Phuengkham, Hitha Rajesh, Quoc D. Mac, Leonard C. Rogers, Aaron D. Silva Trenkle, Swapnil Subhash Bawage, Robert Hincapie, Zhonghan Li, Sofia Vainikos, Inho Lee, Min Xue, Peng Qiu, M. G. Finn, Gabriel A. Kwong. “AND-gated protease-activated nanosensors for programmable detection of anti-tumour immunity.” Nature Nanotechnology (January 2025). https://doi.org/10.1038/s41565-024-01834-8
Funding: This research was supported in part by National Institutes of Health (NIH) grants 5U01CA265711, 5R01CA237210, 1DP2HD091793, and 5DP1CA280832.
News Contact
Jerry Grillo
Jun. 12, 2024
Adoptive T-cell therapy has revolutionized medicine. A patient’s T-cells — a type of white blood cell that is part of the body’s immune system — are extracted and modified in a lab and then infused back into the body, to seek and destroy infection, or cancer cells.
Now Georgia Tech bioengineer Ankur Singh and his research team have developed a method to improve this pioneering immunotherapy.
Their solution involves using nanowires to deliver therapeutic miRNA to T-cells. This new modification process retains the cells’ naïve state, which means they’ll be even better disease fighters when they’re infused back into a patient.
“By delivering miRNA in naïve T cells, we have basically prepared an infantry, ready to deploy,” Singh said. “And when these naïve cells are stimulated and activated in the presence of disease, it’s like they’ve been converted into samurais.”
Lean and Mean
Currently in adoptive T-cell therapy, the cells become stimulated and preactivated in the lab when they are modified, losing their naïve state. Singh’s new technique overcomes this limitation. The approach is described in a new study published in the journal Nature Nanotechnology.
“Naïve T-cells are more useful for immunotherapy because they have not yet been preactivated, which means they can be more easily manipulated to adopt desired therapeutic functions,” said Singh, the Carl Ring Family Professor in the Woodruff School of Mechanical Engineering and the Wallace H. Coulter Department of Biomedical Engineering.
The raw recruits of the immune system, naïve T-cells are white blood cells that haven’t been tested in battle yet. But these cellular recruits are robust, impressionable, and adaptable — ready and eager for programming.
“This process creates a well-programmed naïve T-cell ideal for enhancing immune responses against specific targets, such as tumors or pathogens,” said Singh.
The precise programming naïve T-cells receive sets the foundational stage for a more successful disease fighting future, as compared to preactivated cells.
Giving Fighter Cells a Boost
Within the body, naïve T-cells become activated when they receive a danger signal from antigens, which are part of disease-causing pathogens, but they send a signal to T-cells that activate the immune system.
Adoptive T-cell therapy is used against aggressive diseases that overwhelm the body’s defense system. Scientists give the patient’s T-cells a therapeutic boost in the lab, loading them up with additional medicine and chemically preactivating them.
That’s when the cells lose their naïve state. When infused back into the patient, these modified T-cells are an effective infantry against disease — but they are prone to becoming exhausted. They aren’t samurai. Naïve T-cells, though, being the young, programmable recruits that they are, could be.
The question for Singh and his team was: How do we give cells that therapeutic boost without preactivating them, thereby losing that pristine, highly suggestable naïve state? Their answer: Nanowires.
NanoPrecision: The Pointed Solution
Singh wanted to enhance naïve T-cells with a dose of miRNA. miRNA is a molecule that, when used as a therapeutic, works as a kind of volume knob for genes, turning their activity up or down to keep infection and cancer in check. The miRNA for this study was developed in part by the study’s co-author, Andrew Grimson of Cornell University.
“If we could find a way to forcibly enter the cells without damaging them, we could achieve our goal to deliver the miRNA into naïve T cells without preactivating them,” Singh explained.
Traditional modification in the lab involves binding immune receptors to T-cells, enabling the uptake of miRNA or any genetic material (which results in loss of the naïve state). “But nanowires do not engage receptors and thus do not activate cells, so they retain their naïve state,” Singh said.
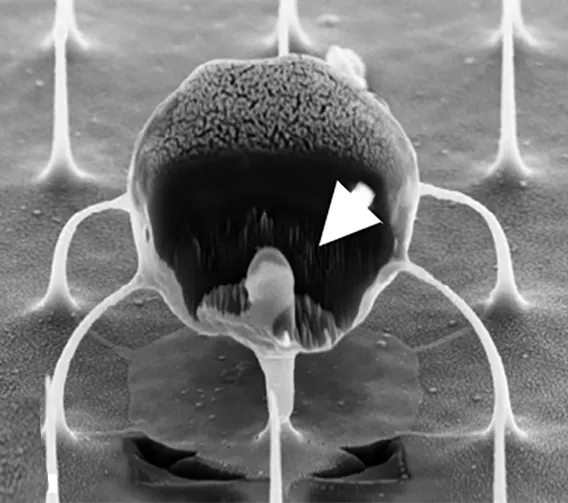
The nanowires, silicon wafers made with specialized tools at Georgia Tech’s Institute for Electronics and Nanotechnology, form a fine needle bed. Cells are placed on the nanowires, which easily penetrate the cells and deliver their miRNA over several hours. Then the cells with miRNA are flushed out from the tops of the nanowires, activated, eventually infused back into the patient. These programmed cells can kill enemies efficiently over an extended time period.
“We believe this approach will be a real gamechanger for adoptive immunotherapies, because we now have the ability to produce T-cells with predictable fates,” says Brian Rudd, a professor of immunology at Cornell University, and co-senior author of the study with Singh.
The researchers tested their work in two separate infectious disease animal models at Cornell for this study, and Singh described the results as “a robust performance in infection control.”
In the next phase of study, the researchers will up the ante, moving from infectious disease to test their cellular super soldiers against cancer and move toward translation to the clinical setting. New funding from the Georgia Clinical & Translational Science Alliance is supporting Singh’s research.
CITATION: Kristel J. Yee Mon, Sungwoong Kim, Zhonghao Dai, Jessica D. West, Hongya Zhu5, Ritika Jain, Andrew Grimson, Brian D. Rudd, Ankur Singh. “Functionalized nanowires for miRNA-mediated therapeutic programming of naïve T cells,” Nature Nanotechnology.
FUNDING: Curci Foundation, NSF (EEC-1648035, ECCS-2025462, ECCS-1542081), NIH (5R01AI132738-06, 1R01CA266052-01, 1R01CA238745-01A1, U01CA280984-01, R01AI110613 and U01AI131348).
News Contact
Jerry Grillo