Improving Precision Oncology Using Multiplexed Bioimages and Artificial Intelligence
May 04, 2020 — Atlanta, GA
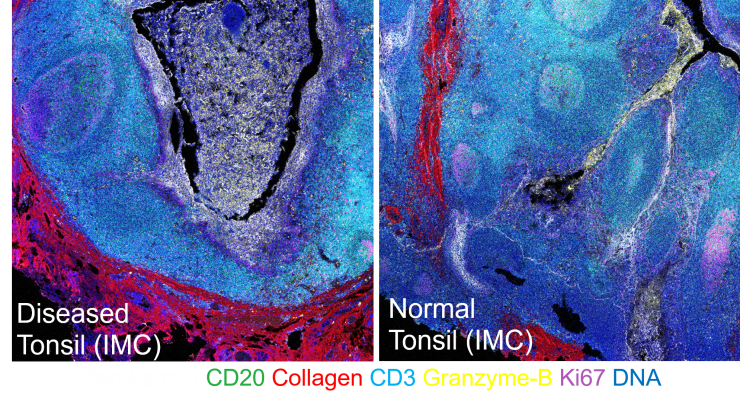
Creating better personalized, precision cancer treatments that are designed around an individual’s functional molecular profile could help predict and deliver more effective treatments for individual patients.
Ahmet Coskun, assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University and the director of Single Cell Biotechnology Laboratory, proposes using multiplexed imaging technologies combined with artificial intelligence to create richer data-driven (molecular) patient profiles at the single-cell level. His detailed explanation of using this approach in biomedical applications was recently published by npj Precision Oncology on May 1, 2020.
A person’s unique molecular profile is compared against a library of other profiles. Each patient profile would be modeled using a variety of the latest imaging, diagnostic, and computational biomedical technologies and procedures used to examine the patient. Ultimately, better personalized treatments could be chosen based on additional multiplexed imaging data gathered from a patient and the increased knowledge accumulated from the application of artificial intelligence upon improved molecular profiles and past patient treatment responses.
“Today’s diagnostic practice relies on histology, a technique to study one biomarker at a time. However, our precision oncology approach visualizes and quantifies hundreds of disease-relevant molecular information in a single test that is routinely performed in a patient’s biopsy specimen,” said Coskun.
Coskun’s method involves using or not using a top-down and bottom-up approach that might be applied to several areas of study. One example includes the collection of single cell and subcellular data. Here, the top-down approach collects human tissue biopsies and studies an individual’s unique tumor characteristics from human tissue biopsies—this also maintains the architecture and spatial distribution of tumor cells and the unique cellular environment around them.
The bottom-up approach generates reproducible data by programming the dynamics of cell-cell interactions on a simple experimental system that mimics the tumor microenvironment in controllable conditions such as cell cultures.
Afterwards, multiplex imaging techniques can then be applied to both approaches to profile the detailed molecular characteristics of an individual. In the example above, the following technologies can help build that profile: immunohistochemistry, immunofluorescence, fluorescence in situ hybridization, multiplexed ion beam imaging, and imaging mass cytometry.
His paper provides additional examples and explanations where a top-down, bottom-up approach is more useful or less useful such as organs-on-chips. He states that organs-on-chips are superior to conventional bottom-up approaches as it recapitulates more features related to tissue architecture and multicellular interaction. Organ-on-chip can also benefit from the power of multiplexed imaging to visualize spatial maps of how cells are organized and to analyze the distribution of certain cells.
From molecular spatial-omics maps, and proteomic, genomic and metabolic profiles
generated by using the top-down, bottom-up, and other cell-based data collection methods, artificial intelligence can then categorize responder and non-responder patient groups to better predict individualized therapeutic treatments for patients.
“Our lab now applies this exciting precision oncology framework to lung and renal cancers in collaboration with clinicians at Emory University School of Medicine. While the current molecular profiling biotechnologies are benchtop and relatively complex to operate, we envision a printer-sized device that can profile spatially resolved multi-omics data in a clinician’s office with a simple user-interface to provide a personalized recommendation for cancer diagnosis and therapeutic planning,” said Coskun.
Additional authors of the paper include Mayar Allam and Shuangyi Cai who are graduate researchers in the lab of Ahmet Coskun.
Ahmet F. Coskun holds a Career Award at the Scientific Interface from Burroughs Wellcome Fund and National Institute of Health K25 Career Development Award (K25AI140783). Coskun’s lab was supported by start-up funds from the Georgia Institute of Technology and Emory University. This material is based upon work supported by the National Science Foundation under Grant No. EEC-1648035 as part of the Cell Manufacturing Technologies (CMaT) Center at Georgia Tech. Ahmet Coskun is affiliated with Georgia Tech’s Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory, and Georgia Tech’s Parker H. Petit Institute for Bioengineering and Bioscience.
Media Contact:
Walter Rich
Communications Manager
Wallace H. Coulter Department of Biomedical Engineering
Georgia Institute of Technology

Walter Rich




