Dec. 10, 2020
The Georgia Tech Supply Chain and Logistics Institute (SCL) is the largest such group in the world, and it provides researchers with many opportunities to help solve global supply chain and logistics problems. The latest addition is the SIReN (Sentient Immersive Response Networks) Lab, dedicated to research leveraging immersive technologies to enhance human capabilities for engineering and managing supply chains and logistic systems.
The SIReN Lab is an associate international laboratory, the result of a partnership between SCL’s Physical Internet Center and IMT Mines Albi, part of the Mines-Telecom Institute in France. The two organizations have historically collaborated on research surrounding artificial intelligence and its interface with these immersive technologies. The SIReN Lab is an extension and formalization of that relationship.
The U.S. arm of the lab is housed in the H. Milton Stewart School of Industrial and Systems Engineering (ISyE) and is directed by Benoit Montreuil, Coca-Cola Material Handling & Distribution Chair and professor in ISyE. Montreuil is also co-director
of SCL and director of the Physical Internet Center. The French lab is led by Frederick Benaben, head of the Interoperability of Organizations research team at IMT Mines Albi. Because of the virtual nature of the work, it is possible to have researchers from both labs working on the same experiment, in the same environment, at the same time.
SIReN Lab research is centered around four main types of response networks — demand, health, humanitarian, and crisis — and the human response to them. A demand response network focuses on how the supply network responds to demand and how to prepare for this response, rather than the other way around. The health and humanitarian response networks, which have become increasingly visible due to the Covid-19 pandemic, relate to issues like disaster recovery and various healthcare supply chains.
The French lab has a significant emphasis on crisis response networks, in which a group of people work together to respond to a crisis in a smart, fair, and efficient manner.
“We currently have a crisis management project where 10 people in France and a few in the U.S. are working together at the same time in a digital twin environment,” said Benaben. “For example, we can have everyone in a building where they can fight a fire, but we can also have some of them in a virtual control room exchanging ideas and making decisions. The options are limitless.”
Researchers are using tools such as dashboards, simulations, games, and in some cases virtual or augmented reality to allow participants to see — and in some cases experience — a vivid picture of a situation with other players in the network.
“In augmented reality, we reinforce what participants see with facts, maps, graphs, and other information that enhance what they are experiencing,” explained Montreuil. “In virtual reality, we project the user into a virtual world, which can be a very vivid representa-tion of the current world, or it can be an abstract world. It can be a very powerful tool.”
“When we put someone in an environ-ment where they can touch, learn, train, experiment, and ultimately decide, it changes the way they approach the problem,” added Benaben.
The French lab launched on Nov. 15, 2019. While the spring 2020 launch of the U.S. lab was postponed due to the Covid-19 pandemic, the team already has several projects underway and is fully operational. Eventually, they would like to see additional SIReN labs join the network to further scale the work being conducted.
“We want to become a global leader in making response networks become more sentient and immersive,” said Montreuil. “This is an exciting new approach that we are bringing to ISyE and to the domain.”
News Contact
Laurie Haigh
Communications Manager
Dec. 03, 2020
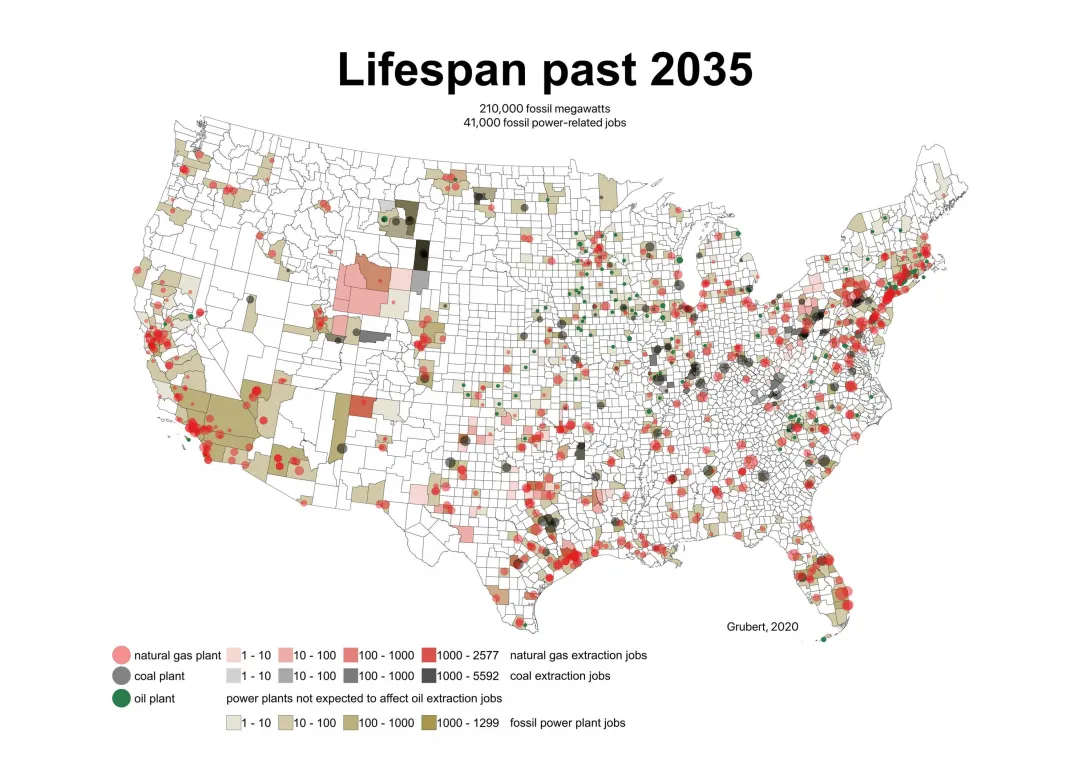
Decarbonizing U.S. electricity production will require both construction of renewable energy sources and retirement of power plants now operated by fossil fuels. A generator-level model described in the Dec. 4 issue of the journal Science suggests that most fossil fuel power plants could complete normal lifespans and still close by 2035 because so many facilities are nearing the end of their operational lives.
Meeting a 2035 deadline for decarbonizing U.S. electricity production, as proposed by the incoming U.S. presidential administration, would eliminate just 15% of the capacity-years left in plants powered by fossil fuels, says the article by Emily Grubert, a Georgia Institute of Technology researcher. Plant retirements are already underway, with 126 gigawatts of fossil generator capacity taken out of production between 2009 and 2018, including 33 gigawatts in 2017 and 2018 alone.
“Creating an electricity system that does not contribute to climate change is actually two processes — building carbon-free infrastructure like solar plants, and closing carbon-based infrastructure like coal plants,” said Grubert, an assistant professor in Georgia Tech’s School of Civil and Environmental Engineering. “My work shows that because a lot of U.S. fossil fuel plants are already pretty old, the target of decarbonization by 2035 would not require us to shut most of these plants down earlier than their typical lifespans.”
Of U.S. fossil fuel-fired generation capacity, 73% (630 out of 840 gigawatts) will reach the end of its typical lifespan by 2035; that percentage would reach 96% by 2050, she says in the Policy Forum article published in Science. About 13% of U.S. fossil fuel-fired generation capacity (110 gigawatts) operating in 2018 had already exceeded its typical lifespan.
Because typical lifespans are averages, some generators operate for longer than expected. Allowing facilities to run until they retire is thus likely insufficient for a 2035 decarbonization deadline, the article notes. Closure deadlines that strand assets relative to reasonable lifespan expectations, however, could create financial liability for debts and other costs. The research found that a 2035 deadline for completely retiring fossil fuel-based electricity generators would only strand about 15% (1,700 gigawatt-years) of capacity life, along with about 20% (380,000 job-years) of direct power plant and fuel extraction jobs that existed in 2018.
In 2018, fossil fuel facilities operated in 1,248 of 3,141 counties, directly employing about 157,000 people at generators and fuel extraction facilities. Plant closure deadlines can improve outcomes for workers and host communities — providing additional certainty, for example, by enabling specific advance planning for things like remediation, retraining for displaced workers, and revenue replacements.
“Closing large industrial facilities like power plants can be really disruptive for the people who work there and live in the surrounding communities,” Grubert said. “We don't want to repeat the damage we saw with the collapse of the steel industry in the 1970s and ’80s, where people lost jobs, pensions, and stability without warning. We already know where the plants are, and who might be affected. Using the 2035 decarbonization deadline to guide explicit, community grounded planning for what to do next can help, even without a lot of financial support.”
Planning ahead will also help avoid creating new capital investment that may not be needed long-term. “We shouldn't build new fossil fuel power plants that would still be young in 2035, and we need to have explicit plans for closures both to ensure the system keeps working and to limit disruption for host communities,” she said.
Underlying policies governing the retirement of fossil fuel-powered facilities is the concept of a “just transition” that ensures material well-being and distributional justice for individuals and communities affected by a transition from fossil to non-fossil electricity systems. Determining which assets are “stranded,” or required to close earlier than expected, is vital for managing compensation for remaining debt or lost revenue, Grubert said in the article.
CITATION: Emily Grubert, “Fossil electricity retirement deadlines for a just transition” (Science, 2020). https://science.sciencemag.org/content/370/6521/1171
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (404-894-6986) (jtoon@gatech.edu)
Writer: John Toon
News Contact
John Toon
Research News
(404) 894-6986
Nov. 30, 2020
When one or more coronavirus vaccines receives FDA emergency use authorization, it will launch a public health and logistics initiative unlike any in U.S. history.
Hundreds of millions of doses will have to distributed nationwide and kept cold until healthcare professionals can administer not one, but two doses to each person. And enough skeptical members of the population will have to be persuaded to receive the vaccine to slow virus transmission.
Beyond those challenges, the distribution effort will have to adapt to unexpected and uneven demand; accommodate recipients who may not return on time for a second dose; train hundreds of thousands of staff from clinics, pharmacies, doctor’s offices, and hospitals; prioritize serving high-risk groups first while encouraging others to wait — all while under tremendous pressure to get the much-anticipated vaccines into use as case counts and the death toll continue rising.
“Time is of the essence because the virus is already so widespread,” said Pinar Keskinocak, the William W. George Chair and professor in the H. Milton Stewart School of Industrial and Systems Engineering (ISyE) and director of the Center for Health and Humanitarian Systems at the Georgia Institute of Technology. “With the pressure on our timeline, knowledge of how quickly the disease is spreading, and the broad U.S. and global need, I can’t think of a comparable public health initiative that has ever been undertaken.”
Shipping and Keeping Hundreds of Millions of Doses Cold
Three vaccines, produced by Moderna, Pfizer and its German partner BioNTech, and Oxford-AstraZeneca, are expected to be available first. The Pfizer-BioNTech vaccine will need to be kept ultra-cold — minus 94 degrees Fahrenheit — on its journey to individual Americans. The Moderna drug won’t have such demanding conditions, but both it and the Pfizer vaccine will tax the existing “cold chain” that keeps vaccines and other temperature-sensitive products in a narrow range of conditions during transport and storage.
The Oxford-AstraZeneca vaccine will have much less stringent requirements and faster ramp-up in capacity, though early testing suggests its efficacy may be lower than the others. That will create tradeoffs between efficacy versus access and speed in distribution.
Plans already exist to get the vaccines from manufacturers to the states, each of which has developed its own distribution plan. Keskinocak worries mostly about “last mile” plans — getting the vaccines to where they will be injected — and getting individuals to those locations.
“Access is going to be a challenge,” she said. “You may be able to get it to locations where it can be distributed, but you have to make sure the people who really need the vaccine can easily access those locations.”
Cold chain transportation, tracking, tracing, and storage already exist in most areas, but refrigeration could be challenging for rural areas that may be at the end of the chain, especially for the vaccine requiring very cold temperatures beyond the capability of freezers found in most doctor’s offices and clinics. And cold can sometimes be too cold, Keskinocak said.
“We often think about keeping it cold, but sometimes it may be too cold, which is not good. It’s not just whether the temperature exceeded the required level, but also whether it went below that. It is important to keep the vaccine exactly at the required temperature level.”
Pfizer has developed a shipping container that includes a temperature tracking device — and 50 pounds of dry ice to maintain the right temperature during transit. Because it is contained in small vials and the liquid vaccine is diluted for use, the overall volume being shipped will be relatively small, limiting the number of packages that will be moved and stored, Keskinocak noted.
Ultimately, the cold chain may play a significant role in vaccine effectiveness. Currently, the vaccines being produced by Pfizer/BioNTech and Moderna are reported to have a higher efficacy than the Oxford-AstraZeneca vaccine — but only if they can be maintained at the proper temperatures. The timing, magnitude, and duration of temperature fluctuations during transport and before administration could affect that in ways that may be difficult to assess.
“Our current modeling shows that a vaccine that is less effective but that can be distributed more quickly and more widely might work better in some settings than a more effective vaccine, thereby reducing the total number of infections in the population,” Keskinocak said.
If You Build It, Will They Come?
Expectations are that the nation is hungry for a vaccine to escape the horrors of Covid-19. But a recent Gallup survey shows that only 58% of respondents said they planned to receive the vaccine when it becomes available. Boosting that percentage will require a massive communications effort to overcome vaccine reluctance and concerns fueled by the uneven nature of the U.S. pandemic response.
“If we can get the vaccine to locations where people can access it, and we have the necessary syringes, supplies, and PPE, as well as the healthcare staff to administer the injections, it’s not clear that people will come to receive it in large enough numbers,” Keskinocak said. “That’s one major component missing from a lot of the plans that I see at the state level.”
The communications program will have to run in parallel to the vaccine distribution, and they have to be coordinated so that supply meets demand.
“Public health communication and dissemination of information at the right time and in the right language is going to be at least as important and challenging as the logistics of distributing the vaccine,” Keskinocak said. “Communication is going to shape demand to a large extent. If one is more effective than the other, we will have a mismatch between demand and supply.”
Different demographic populations have different levels of trust for medicine in general and vaccines in particular, she said, so communications campaigns will have to focus on issues of concern to those groups. Unexpected variations in vaccine demand caused by these concerns could also create logistical uncertainties.
“We can try to forecast demand, and ship supplies to those locations,” she said. “But historically, we have seen that demand can exceed supply in one location while inventory builds up in another location. We need to avoid this situation of unmet demand and unused vaccine.”
Another issue will be the two doses necessary for the vaccine. The second dose must be received within a narrow range of time for the two-dose vaccine to be effective. Should a second dose be reserved for every person receiving a first dose, or should the goal be to get as many doses out as possible?
“Some people may never show up to be vaccinated, while others will receive the first dose, but may not come back for the second dose,” she said.
Getting the Program Started
The first available doses will likely go to healthcare workers and first responders who are on the front lines of battling Covid-19. That is expected to be the easier part of vaccination logistics, and the lessons learned there should help with the much more massive vaccination campaign for high-risk individuals and the general public.
As vaccine production and distribution capacity ramp up, other groups will be next in line. While distributing small batches as manufacturers produce it can create some supply challenges, that also allows the system to more easily adjust to unexpected demand.
Even though distributing and administering vaccines is something the U.S. healthcare system does routinely, the size and timeline of this project are unprecedented, Keskinocak noted.
Beyond the logistical and communications needs, the vaccination program will also have a strong information technology component. Administration will likely be by appointment, and each injection will have to be reported to a vaccine registry to provide a record of which vaccines people have received and when.
Vaccinating People Who May Already Be Immune
It’s estimated that the number of reported Covid-19 cases may be just 10% of the actual number of infections in the U.S. Assuming recovery from the virus confers immunity for some period of time means there may be quite a few people who don’t actually need the vaccine right away to be protected. But there are currently no plans to determine whether recipients are already immune before they receive the vaccine.
“There are a lot of people out there who have some level of immunity to the coronavirus,” Keskinocak said. “The plans I’ve seen don’t include the serological testing that would be needed to identify people with some level of immunity, which could be around 30% of the population by the time the vaccine gets out to the general public.”
Testing for immune antibodies could be done ahead of the vaccination program, but that would create an extra step in a process that is already quite complicated. Healthcare systems such as the U.S. Department of Veterans Affairs or certain private insurance plans could include that step, especially if vaccine supplies lag behind demand.
“The big complexity is timing,” she said. “Once vaccines become available, you’ll want to deliver them as quickly as possible to as many people as possible in a very short time frame.”
Annual vaccination campaigns for the seasonal flu set ambitious goals for the population levels they want to reach, but the time challenges will be much greater for the coronavirus vaccine.
“The seasonal flu vaccine becomes available months before the virus spreads broadly, so we have quite a bit of time to administer it before we get into the peak of the flu season,” she said. “We have been in the midst of the Covid-19 pandemic for several months now. We are really late in the game, so we don’t have the luxury of time.”
Keskinocak is cautiously optimistic that the challenges will ultimately be addressed.
“There are certainly still lots of unknowns,” she said. “But the state plans I have seen look reasonable from a supply chain standpoint. Some of the decisions will be made once the states receive the vaccine, and exactly how they do it will be somewhat up to the local jurisdictions. There are still many things that need to be decided to make this unprecedented initiative live up to its goals.”
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (404-894-6986) (jtoon@gatech.edu)
Writer: John Toon
News Contact
John Toon
Research News
(404-894-6986)
Nov. 18, 2020
Georgia Institute of Technology is a governing member of the BioIndustrial Manufacturing and Design Ecosystem (BioMADE), a nonprofit that recently won a seven-year, $87 million award from the U.S. Department of Defense (DoD).
Created by the Engineering Biology Research Consortium, BioMADE will collaborate with public and private entities to advance sustainable and reliable bioindustrial manufacturing technologies. Headquartered at the University of Minnesota in St. Paul, BioMADE includes some of the largest bioindustrial manufacturing employers in the U.S. working in conjunction with some of the top educators in the world.
In support of this collaboration, the $87 million in DoD funding will be combined with more than $187 million in non-federal cost-share from 31 companies, 57 colleges and universities, six nonprofits, and two venture capital groups across 31 states.
Pamela Peralta-Yahya, an associate professor in Georgia Tech’s School of Chemistry and Biochemistry and School of Chemical and Biomolecular Engineering, is Tech’s representative to BioMADE’s Leadership Council, which will set the organization’s funding priorities.
Peralta-Yahya says, “An incredible cross section of Georgia Tech faculty contributed to the BioMADE proposal; over 30 faculty members, spanning five Schools across the College of Science, College of Engineering, and the Ivan Allen College of Liberal Arts.”
She notes: “Georgia Tech’s involvement in BioMADE is poised to catalyze interdisciplinary collaborations across the university, from data science and downstream processing to supply chain logistics and the policy, legal, and biosafety implications of bioindustrial applications. The projects funded by BioMADE will give undergraduates and graduate students a springboard to the emerging biomanufacturing and related areas.”
Mark Styczynski, an associate professor in Georgia Tech’s School of Chemical and Biomolecular who is Tech’s representative to the BioMADE Technical Committee, says: “Georgia Tech will be a member of BioMADE at the governing level, the highest level of engagement for academic institutions. We are excited about the resulting opportunities for Georgia Tech to bring to bear its manufacturing, chemical, and biochemical expertise on new applications and focus areas in the biomanufacturing space.”
He adds: “Our involvement in this area is a great complement to other biomanufacturing efforts at Georgia Tech and will contribute to a rapidly growing bioeconomy in Georgia.”
Through a close relationship with DoD and the Military Services, BioMADE will work to establish long-term and dependable bioindustrial manufacturing capabilities for a wide array of products. Anticipated bioindustrial manufacturing applications include the following products: chemicals, solvents, detergents, reagents, plastics, electronic films, fabrics, polymers, agricultural products (e.g. feedstock), crop protection solutions, food additives, fragrances, and flavors.
BioMADE’s efforts will examine and advance industry-wide standards, tools, and measurements; mature foundational technologies; foster a resilient bioindustrial manufacturing ecosystem; advance education and workforce development; and support the establishment and growth of supply chain intermediaries that are essential for a robust U.S. bioeconomy. Other important focus areas include challenges related to biosafety and security and ethical, legal, and societal considerations.
Stefan France, an associate professor in Georgia Tech’s School of Chemistry and Biochemistry is Tech’s representative to BioMADE’s Education and Workforce Committee, which will help craft and implement the organization’s strategic plan.
France explains that this committee “will concentrate its efforts in three major areas: curriculum and training for the bioindustrial workforce, promoting awareness of career opportunities, and coordination across the STEM community, the biomanufacturing ecosystem, and the training pipeline—everything from K-12 to community and technical colleges to four-year colleges, graduate programs, and post-graduate training.”
News Contact
Brad Dixon, braddixon@gatech.edu
Nov. 17, 2020
Jialei Chen, an industrial and systems engineering (ISyE) doctoral student and graduate research assistant in the Georgia Tech Manufacturing Institute (GTMI), won two Best Student Paper Awards at this year’s 2020 INFORMS Conference. The annual INFORMS conference on business analytics and operations research brings together nearly 1,000 leading analytics professionals and industry experts to share ideas, network and learn about a range of current topics and trends that can help businesses and organizations improve their analytics prowess by applying science to the art of business.
Chen won Best Student Paper Award in the Quality, Statistics, and Reliability track for “Adaptive Design for Gaussian Process Regression under Censoring.” This paper presented an experimental design and modeling method for censored physical experiments. Censoring is commonly encountered in experimentation due to the limits in a measurement device, safety considerations of the experimenter, and a fixed experimental time budget. To tackle this, he proposed a novel adaptive design method, which first estimates the possibility of censoring and then adaptively chooses design points to minimize predictive uncertainty under censoring. He demonstrates the effectiveness of the proposed method in two real-world applications on surgical planning and wafer manufacturing.
Chen won Best Student Paper Runner-up Award in the Data Mining track for “APIK: A Physics-Informed Kriging Model with Partial Differential Equations.” This paper presented a learning framework that combines limited data and the auxiliary partial differential equations. One of the key challenges in applying state-of-the-art machine learning methods in real-world engineering applications is that the available measurement data is scarce. In this work, he proposed to incorporate the auxiliary partial differential equations in the learning model and therefore improve the predive performance. The proposed APIK model can leverage linear and nonlinear PDEs and enjoy simple and closed-form prediction and uncertainty quantification. He applied the proposed method to two real-world applications on flow dynamics and thermal processes.
Chen’s advisors for both papers were Georgia Tech ISyE professors Chuck Zhang and Roshan Joseph.
“I’m honored to have won best student paper award in the quality, statistics, and reliability track at INFORMS 2020, and to have another paper win second place in the data mining track,” said Chen. “My research focuses on engineering-driven learning methodologies, and data-driven modeling for complex engineering and manufacturing systems. The two awards are a great encouragement for me and inspire me to accomplish more in-depth and impactful works in the future. I would like to express my highest gratitude to my supervisors, professors Chuck Zhang and Roshan Joseph. I would also like to thank the support and assistance from GTMI, which helped to make the two projects possible.”
News Contact
Walter Rich
Nov. 13, 2020
Industrial Internet of Things in 2020 was presented by Alain Louchez at the IoT for Manufacturing Symposium organized by the Factory Information Systems Center at the Georgia Tech Manufacturing Institute on November 11, 2020.
For a full activity report please see: https://cdait.gatech.edu/Activities/Industrial_Internet_of_Things
Oct. 07, 2020
California, September 14, 2020: TiE Global hosted the 2nd edition of the TiE University Pitch Competition over the weekend. Cash prizes of close to $14,000 and almost $35,000 worth of in-kind prizes were given out to the 13 participants. TiE Atlanta’s Aerodyme Technologies from Georgia Institute of Technology came in first, winning $5000. TiE Silicon Valley’s team Ambii from San Jose State University bagged the second prize, winning $3000; while TiE Toronto’s LSK Technologies, a MedTech startup from the University of Toronto emerged as the third winner, winning $2000.
The winning team, Aerodyme Technologies has created a novel device that saves fuel costs for tractor-trailers by minimizing aerodynamic drag. Ambii, which came in second, provides an in-store music streaming platform for retail outlets, cafes and restaurants without the hassles of licensing. Third prize winner, LSK Technologies, created a diagnostic device for testing infectious diseases such as Covid19 at the point of need.
Congratulating the winning team, Mahavir Pratap Sharma, Chairman of the TiE Global Board of Trustees said, "We are proud of these young talented entrepreneurs. Their growth story from pitches at their local TiE Chapters till the Global Finals has seen a massive expansion and iteration of thought and structure. This is a complete team effort put forward by the program co-chairs and charter member mentors who helped them grow their idea, giving them thorough guidance and mentoring the students over the last few months to compete on a global stage.”
Aerodyme Technologies participated in Georgia Tech’s CREATE-X Startup Launch program during summer 2019. During the startup accelerator program, the Aerodyme team was able to conduct customer discovery and receive mentorship and funding to take their product to market. The team also won 2nd place in the 12th annual InVenture Prize competition in March 2020. As part of the competition, Aerodyme received $10,000 in cash and patent filing assistance funded by the Georgia Tech Research Corporations.
The second edition of the TiE University jointly organized by TiE Atlanta & TiE Hyderabad had 13 teams representing TiE chapters from India, Israel, UAE, Israel, USA, and Canada. These teams were previously the chapter winners and were then mentored to participate in the Global finals. After the Semi-final round on Saturday, seven teams were selected for the finals. Teams had a 10-minute pitch to an eminent Jury panel and a 5-minute live Q&A.
Speaking at the Presentation Ceremony the TiE University program co-chairs Dr. Paul Lopez, SubbaRaju Pericherlaand Viiveck Verma, said they are looking to expand the program participation to 40 chapters and 400+ universities worldwide by 2022.
The worldwide jury panel for the finals, consisting of VCs and investors, included Bodhi Capital’s Dharti Desai, Silicon Valley Bank’s Priya Rajan, Elevate Capital’s Kumar Sripadam, and Inflexor Ventures’ Venkat Vallabhaneni. Semi-finals judges from across the globe included Craig Abbott, Rakesh Bhatia, Radhika Iyengar, Rama Devi Kanneganti, Doc Parghi, and Sonia Weymuller.
Apart from the top three winners, prizes were announced in various other categories. Silicon Valley’s Ambii and New Jersey’s Volant from New Jersey Institute of Technology jointly received a $1,500 Best Elevator Pitch award. Similarly, Teams Clean Electric from IIT/BHU, Varanasi representing TiE Mumbai, and Aruga Technologies from Carnegie Mellon University representing TiE Pittsburgh jointly received $1,500 Best Technology prize. Frinks, from IIT Hyderabad, representing TiE Hyderabad took the $500 People's Choice Award. Apart from this, all participating teams walked away with technology and service prizes worth $2500 per team.
The competition also hosted a keynote address by serial entrepreneur Thejo Kote. Thejo sold his connectivity startup, Automatic for $115 million to SiriusXm. He inspired the university startup teams and online audience alike by saying, “The biggest lesson I’ve learned along the way is to make sure you enjoy the journey (of building a startup) and it’s something that you learn from and grow from. Else it will be a lot more challenging.”
About TiE University:
TiE University program focuses on enhancing the learning objectives of university students globally through the creation and presentation of business pitches for startups and new small business ventures so that they aspire to become entrepreneurs. TiE University is looking to expand its reach to 40 chapters and 400+ universities worldwide. If you’re interested, reach out to the below-mentioned media contact. For more information, visit - https://tie.org/tie-university/
About TiE Global:
TiE Global, is a non-profit organization dedicated to fostering entrepreneurship around the World. TiE strives to inspire entrepreneurs through mentoring, networking, education, incubating and funding programs and activities. With nearly 2000+ events held each year, TiE brings together the entrepreneurial community to learn from local leaders, as well as each other. Few of the annual flagship events conducted by TiE are TYE, TiE Women, TiE University, TiECons and TGS. For more information, please visit our website at https://tie.org/
Media Contact:
Aparna Mishra Aparna@tie.org
Oct. 07, 2020
By Jessica Barber
On Wednesday, September 16, the Office of Undergraduate Education (OUE) hosted the kick-off session for the 13th Annual InVenture Prize. With over $35,000 in prizes, the competition is the holy grail of college entrepreneurship. Although the InVenture Prize officially starts in January 2021, students have already begun their preparations and idea declarations.
Unlike previous years, the kick-off was hosted online through Gatherly, a virtual event platform recently built by none other than Georgia Tech students. Despite this, attendees did not miss a beat. The kick-off marked a return to normalcy for the Georgia Tech innovation community from learning key information about the competition to directly speaking with past winners.
After a welcome from interim Assistant Director of Student Innovation, Recha Reid, students were given an overview of some upcoming InVenture Prize events, including the ongoing Pitch Your Idea and IdeaBuzz sessions. Students were given an overview of OUE’s customer discovery, financial forecasting, marketing, and patent/copyright workshops. From there, the floor was turned over to Dr. Chris Reaves, executive director of the office for Academic Enrichment Programs.
“At its core, the InVenture Prize is an invention startup competition, but we work together — even the teams work with each other — to help one another. We achieve more, grow more, and develop our companies better when we’re helping each other, and that’s a big part of what we’re doing,” Reaves explained.
Later, students were given the opportunity to speak with representatives from Queues and Aerodyme, the respective first- and second-place winners of the 2020 InVenture Prize. Students learned firsthand what it takes to succeed on the InVenture Prize stage; the teams later offered advice on the invention process, their lessons learned, and the visibility benefits of participating in the competition.
“If you’re on the edge right now about doing InVenture Prize, definitely do it. We actually had that same thought before we did it, and we’re just so glad that we did. It’s a lot of work, and you’re going to step outside of your comfort zone, but it’s so worth it”, said Joy Bullington of team Aerodyme Technologies.
Queues team member Sam Porta similarly had some words of encouragement for those looking into the 2021 InVenture Prize.
“The difference between an entrepreneur and someone who’s just engineering something is persistence, and the InVenture Prize is a great opportunity to test this. If you think you’ve come up with something great that has a lot of value, then, by all means, do it,” Porta emphasized.
Towards the end of the session, students were invited to visit virtual “booths” dedicated to areas of health, retail, fintech, transportation, education tools, gaming, and networking.
“InVenture is honestly one of the reasons I chose to come to Tech, and I’m just so excited to come into with something that I’m really confident about,” an attendee said.
“The most interesting thing about tonight was hearing from the past winners and having them talk about their experiences. Definitely super excited to apply, and hopefully we do really well,” another stated.
Registration for the 2021 InVenture Prize will remain open until January. Student innovators are invited to check out OUE’s information and development sessions to be held throughout the Fall semester. All dates and related topics can be found at innovation.gatech.edu and inventureprize.gatech.edu.
FIND OUT MORE ABOUT THE 2021 INVENTURE PRIZE BY CLICKING HERE
Visit us on
Instagram @gtinventure
Twitter @InVenturePrize
Sep. 04, 2020
Imagine a reusable face mask that protects wearers and those around them from SARS-CoV-2, is comfortable enough to wear all day, and stays in place without frequent adjustment. Based on decades of experience with filtration and textile materials, Georgia Institute of Technology researchers have designed a new mask intended to do just that — and are providing the plans so individuals and manufacturers can make it.
The modular Georgia Tech mask combines a barrier filtration material with a stretchable fabric to hold it in place. Prototypes made for testing use hook and eye fasteners on the back of the head to keep the masks on, and include a pocket for an optional filter to increase protection. After 20 washings, the prototypes have not shrunk or lost their shape.
“If we want to reopen the economy and ask people to go back to work, we need a mask that is both comfortable and effective,” said Sundaresan Jayaraman, the Kolon Professor in Georgia Tech’s School of Materials Science and Engineering. “We have taken a science-based approach to designing a better mask, and we are very passionate about getting this out so people can use it to help protect themselves and others from harm.”
The fundamental flaw in existing reusable cloth masks is that they — unlike N95 respirators, which are fitted for individual users — leak air around the edges, bypassing their filtration mechanism. That potentially allows virus particles, both large droplets and smaller aerosols, to enter the air breathed in by users, and allows particles from infected persons to exit the mask.
The leakage problem shows up in complaints about eyeglasses fogging up as exhaled breath leaks around the nose, making people less likely to wear them. The fit problem can also be seen in constant adjustments made by wearers, who could potentially contaminate themselves whenever they touch the masks after touching other surfaces.
To address the leakage challenge, Jayaraman and principal research scientist Sungmee Park created a two-part mask that fastens behind the head like many N95 respirators. The front part — the barrier component — contains the filtration material and is contoured to fit tightly while allowing space ahead of the nose and mouth to avoid breathing restrictions and permit unrestricted speech. Made from the kind of moisture-wicking material used in athletic clothing, it includes a pocket into which a filter can be inserted to increase the filtration efficiency and thereby increase protection. The washable fabric filter is made of a blend of Spandex and polyester.
The second part of the mask is fashioned from stretchable material. The stretchable part, which has holes for the ears to help position the mask, holds the front portion in place and fastens with conventional hook and eyelet hardware, a mechanism that has been used in clothing for centuries.
“We want people to be able to get the mask in the right place every time,” Jayaraman said. “If you don’t position it correctly and easily, you are going to have to keep fiddling with it. We see that all the time on television with people adjusting their masks and letting them drop below their noses.”
Beyond controlling air leakage, designing a better mask involves a tradeoff between filtration effectiveness and how well users can breathe. If a mask makes breathing too difficult, users will simply not use it, reducing compliance with masking requirements.
Many existing mask designs attempt to increase filtration effectiveness by boosting the number of layers, but that may not be as helpful as it might seem, Park said. “We tested 16 layers of handkerchief material, and as we increased the layers, we measured increased breathing resistance,” she said. “While the breathing resistance went up, the filtration did not improve as much as we would have expected.”
“Good filtration efficiency is not enough by itself,” said Jayaraman. “The combination of fit, filtration efficiency, and staying in the right place make for a good mask.”
The stretchable part of the mask is made from knitted fabric — a Spandex/Lyocell blend — to allow for stretching around the head and under the chin. The researchers used a woven elastic band sewn with pleats to cover the top of the nose.
The researchers made their mask prototypes from synthetic materials instead of cotton. Though cotton is a natural material, it absorbs moisture and holds it on the face, reducing breathability, and potentially creating a “petri dish” for the growth of microbes.
“Masks have become an essential accessory in our wardrobe and add a social dimension to how we feel about wearing them,” Park said. So, the materials chosen for the mask come in a variety of colors and designs. “Integrating form and function is key to having a mask that protects individuals while making them look good and feel less self-conscious,” Jayaraman said.
The work of Jayaraman and Park didn’t begin with the Covid-19 pandemic. They received funding 10 years ago from the Centers for Disease Control and Prevention to study face masks during the avian influenza outbreak. Since then Jayaraman has been part of several National Academy of Medicine initiatives to develop recommendations for improved respiratory protection.
Covid-19 dramatically increased the importance of using face masks because of the role played by asymptomatic and pre-symptomatic exposure from persons who don’t know they are infected, Jayaraman said. While the proportion of aerosol contributions to transmission is still under study, they likely increase the importance of formfitting masks that don’t leak.
Jayaraman and Park have published their recommendations in The Journal of The Textile Institute, and will make the specifications and patterns for their mask available to individuals and manufacturers. The necessary materials can be obtained from retail fabric stores, and the instructions describe how to measure for customizing the masks.
“There is so much misinformation about what face masks can do and cannot do,” Jayaraman said. “Being scientists and engineers, we want to put out information backed by science that can help our community reduce the harm from SARS-CoV-2.”
Link to plans, patterns and specifications for this mask
CITATION: Sungmee Park and Sundaresan Jayaraman, “From containment to harm reduction from SARS-CoV-2: a fabric mask for enhanced effectiveness, comfort, and compliance.” (The Journal of The Textile Institute, 2020) https://doi.org/10.1080/00405000.2020.1805971
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (404-894-6986) (jtoon@gatech.edu).
Writer: John Toon
News Contact
John Toon
Research News
(404) 894-6986
Aug. 06, 2020
BioFabUSA, a Department of Defense-funded Manufacturing Innovation Institute within the Manufacturing USA network, has awarded the Georgia Institute of Technology and industry partner Rockwell Automation a project entitled, “Wireless Electrochemical Sensor Capsules for Real-Time Monitoring of Cell Secretomes and Culture Media in Tissue Growth Bioreactors.” Real-time bioprocess monitoring and control is needed for the scalable production and deployment of efficacious tissue engineered medical products (TEMPs) at reasonable cost.
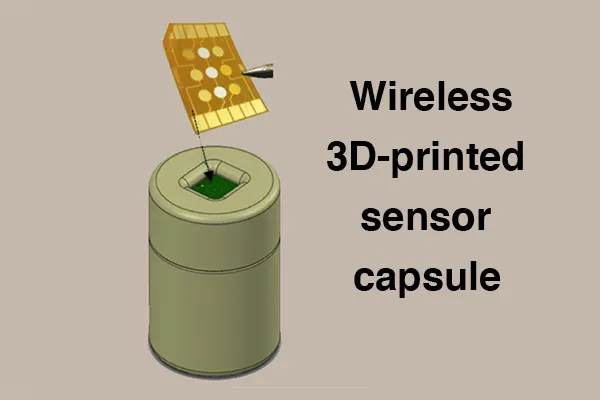
Billyde Brown, the project's principal investigator, explained, “we are addressing this challenge by working with BioFabUSA, our partners at the Georgia Tech School of Materials Science and Engineering, the Marcus Center for Therapeutic Cell Characterization and Manufacturing, as well as Rockwell Automation, to develop a fully integrated, wireless, 3D-printed sensor ‘capsule’ to be used for in-situ multiplexed monitoring of critical quality attributes (CQAs). The targeted CQAs include pH, glucose, lactate, and select secreted biomarker concentrations from human mesenchymal stem cells – one of the most common cell types used in tissue engineering.”
In both biopharmaceutical and regenerative medicine industries, an urgent need remains for in-line sensor technology for quantitative real-time bioprocess monitoring and control. Unfortunately, many key CQAs are still monitored off-line or at-line using destructive testing or technologies of significant complexity and cost. In at-line measurement, the sample is typically withdrawn from a single location in the bioreactor and analyzed in close proximity to the process stream, whereas in off-line measurements, the sample is taken to a laboratory and the results are usually not returned in a timely manner for process control.
The Georgia Tech team has previously developed potentiometric sensors based on an extended gate field-effect-transistor (FET) topology whereby a separate gold electrode surface is functionalized with an analyte-specific layer that selectively reacts or binds with the chemical or biomolecule of interest. The charge associated with the attached analyte results in a potential change of the gold electrode. These sensors have previously been used to detect chemicals such as pH and lactate, as well as specific proteins/antibodies in a laboratory environment with accuracy and dynamic range equivalent to Surface Plasmon Resonance (SPR) and Enzyme-Linked Immunosorbent Assay (ELISA). One of the unique aspects of this system is that each sensor surface can be individually functionalized permitting multiplexed (simultaneous) detection of almost any number of different chemicals/biomolecules of interest.
In this project, the Georgia Tech team will integrate these sensors into a “capsule” device smaller than the size of a golf-ball and packaged in a 3D-printed waterproof and biocompatible polymer. The capsule will contain a multiplexed sensor chip, with sealed opening to facilitate interaction between the sensor chip and tissue culture environment, Li-polymer battery, and electronics for micro-control, data acquisition and wireless transmission of sensor data to the smartphone of a technician in charge of monitoring the bioreactor process. In addition, Georgia Tech will work with Rockwell to develop an IoT platform such that other permitted internet-connected devices can securely access the data via a cloud server. Another unique aspect of this technology is that multiple “capsules” could be deployed within a stirred tank bioreactor during high volume production of medical products with the ability to move efficiently throughout the bioreactor due to the mechanical forces of the impellors. This would allow for unprecedented simultaneous measurements at various points within the bioreactor, giving accurate representations of the homogeneity of key parameters over time thus achieving in-situ monitoring of CQAs with high spatial and temporal resolution.
Georgia Tech project leads include Billyde Brown, Ph.D., Kan Wang, Ph.D., and Eric Vogel, Ph.D. Brown is research faculty and director of manufacturing education programs at the Georgia Tech Manufacturing Institute (GTMI). Wang is lead researcher of additive manufacturing in the Bio-Engineering Research Laboratory at GTMI. Vogel is a professor at the School of Materials Science and Engineering and deputy director for the Institute of Electronics and Nanotechnology at Georgia Tech. The Georgia Tech project leads will also receive support and assistance from Carolyn Yeago, Ph.D., and Krishnendu Roy, Ph.D. whom are directors of the Marcus Center for Therapeutic Cell Characterization and Manufacturing (MC3M). Leading the project for Rockwell Automation is Wayne Charest, who also serves as a liaison between Rockwell and BioFabUSA.
“Being able to obtain real-time data on relevant biomarkers will be critical in advancing the field of tissue engineering,” said Stephanie Robichaud, technical project manager with the Advanced Regenerative Manufacturing Institute. “Getting this important information and being able to react to it quickly will result in more consistent manufacturing of a final product that meets its critical quality attributes.”
About the Georgia Institute of Technology
The Georgia Institute of Technology, also known as Georgia Tech, is one of the nation’s leading research universities — a university that embraces change while continually Creating the Next. The next generation of leaders. The next breakthrough startup company. The next lifesaving medical treatment.
Georgia Tech provides a focused, technologically based education to more than 36,000 undergraduate and graduate students. The Institute has many nationally recognized programs, all top-ranked by peers and publications alike, and is ranked among the nation’s top five public universities by U.S. News & World Report. It offers degrees through the Colleges of Computing, Design, Engineering, Sciences, the Scheller College of Business, and the Ivan Allen College of Liberal Arts. As a leading technological university, Georgia Tech has more than 100 centers focused on interdisciplinary research that consistently contribute vital research and innovation to American government, industry, and business. https://www.gatech.edu/
About Rockwell Automation
Rockwell Automation is the largest company in the world that is dedicated to industrial automation and information and is committed to enabling the next generation of smart manufacturing. Rockwell’s mission is to improve the quality of life by making the world more productive and sustainable.
https://www.rockwellautomation.com
About BioFabUSA
BioFabUSA is a DOD-funded Manufacturing USA Innovation Institute (MII) sustained by the Advanced Regenerative Manufacturing Institute (ARMI), a non-profit organization located in Manchester, New Hampshire. ARMI's mission is make practical the scalable, consistent, cost-effective manufacturing of tissue engineered medical products and tissue-related technologies, to benefit existing industries and grow new ones. https://www.armiusa.org/
Georgia Tech Manufacturing Institute
813 Ferst Drive, NW
Atlanta, GA 30332 USA
Media Relations Contact: Walter Rich (walter.rich@research.gatech.edu)